已传文件:photo/1769581837.png
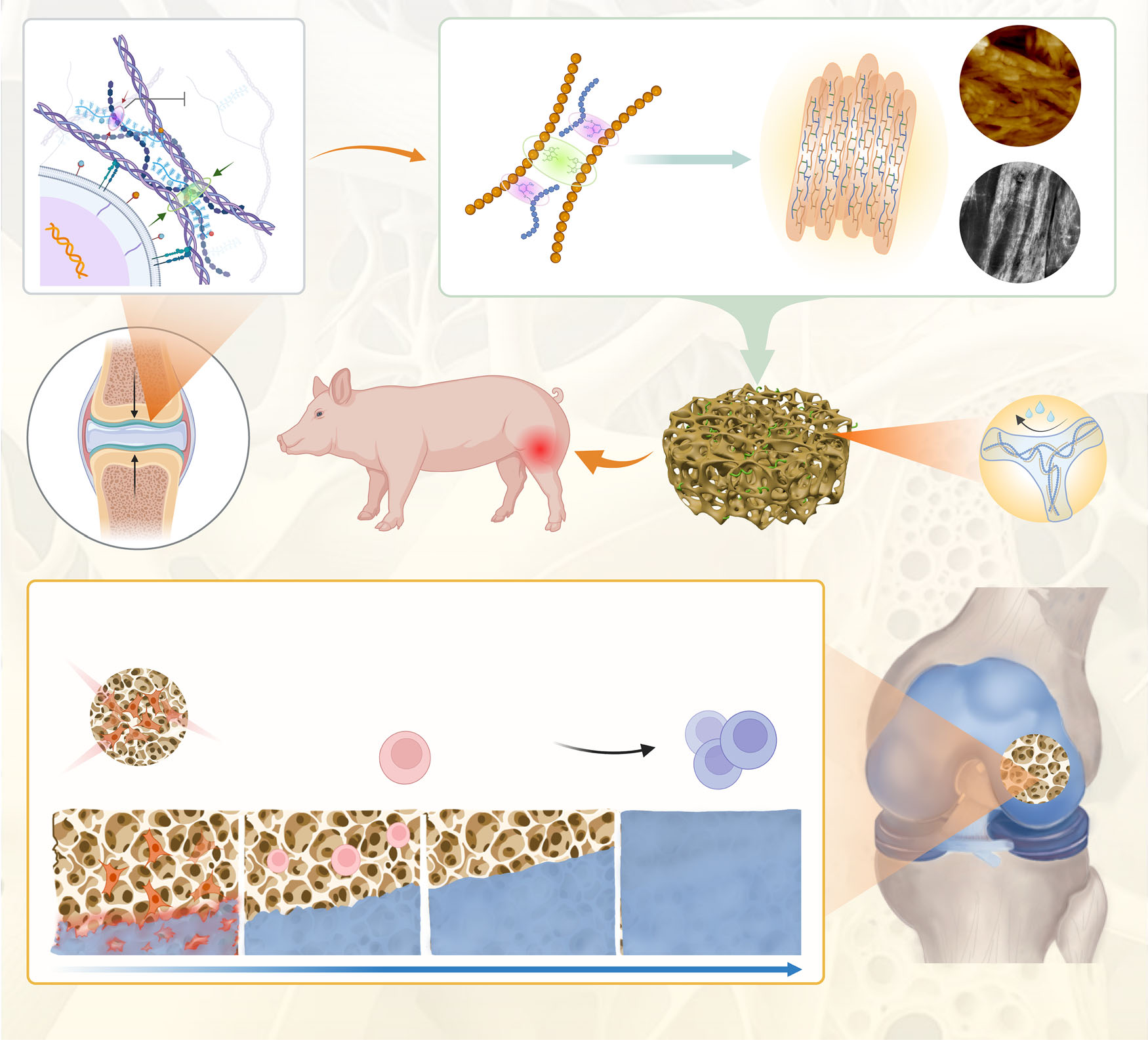
Poor structural stability of cartilage implants and insufficient endogenous activation may be key factors in cartilage regeneration after microfracture. In this book, inspired by the restrictive effect of collagen fibers on proteoglycans, we developed artificial proteoglycan assemblies (DSPG@Pep) by sequentially co-assembling bioactive polysaccharides, proteins, and peptides through electrostatic interactions and covalent conjugation.The DSPG@Pep exhibits anti-swelling, anti-compression, and anti-degradation properties, significantly activating endogenous stem cell recruitment and direct chondrogenic differentiation. Specifically, DSPG@Pep downregulates calcium signaling pathways (such as CACNA1G, AVPR1A, etc.) and ECM receptor interactions (IBSP and COL4A2), thereby reducing the tendency for ossification (EFEMP1, SCUBE3, and SPARCL1), manifested by decreased cytoplasmic calcium concentration and β1 integrin aggregation. Cartilage defect models in male rabbits and pigs confirmed that DSPG@Pep can be stably fixed at defect sites, promoting structural and functional remodeling of new cartilage. These findings provide a promising biomaterial design strategy for endogenous cartilage regeneration.

Microfracture surgery is a common clinical method for repairing articular cartilage defects, but the regeneration it induces is mostly fibrocartilage, which has poor mechanical properties and is prone to degeneration. The insufficient structural stability of the implanted material itself and the limited capability for endogenous activation may be key factors affecting effective cartilage regeneration after surgery. In natural cartilage, the collagen fiber network works synergistically with aggregates of proteoglycans, not only providing the tissue with good mechanical and lubricating properties, but more importantly, the collagen fibers can limit the excessive swelling of proteoglycans due to water absorption, thereby maintaining the structural stability of the matrix. However, this natural stabilization mechanism has not been adequately referenced or applied in previous designs of biomimetic artificial cartilage materials.
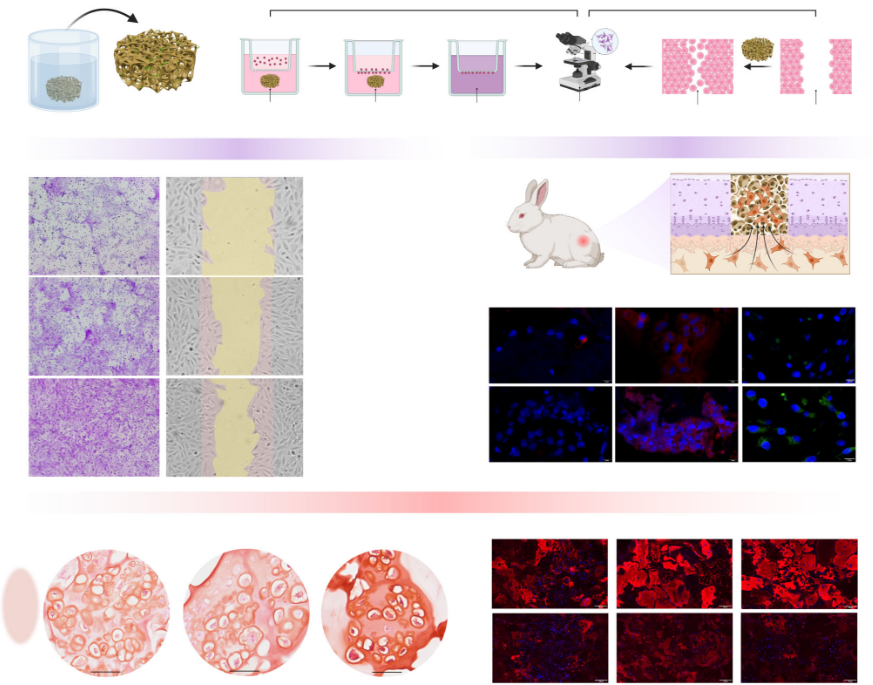
Inspired by this, the research team successfully constructed an anti-swelling artificial proteoglycan assembly with self-activation capabilities through a stepwise co-assembly strategy at the molecular level. This material uses polyphenol-modified silk fibroin and thiolated hyaluronic acid as basic assembly units, first forming precursors through electrostatic interactions, and then undergoing chemical cross-linking under pH regulation to create a stable network with a fibrous bundle structure. Furthermore, by attaching active peptides, the material is endowed with the biological function of actively recruiting endogenous stem cells and guiding their differentiation into cartilage. Experiments showed that this artificial proteoglycan assembly exhibited excellent anti-swelling, compression-resistant, and degradation-resistant properties, with mechanical performance significantly superior to that of control materials without the biomimetic stabilization mechanism.
In vitro cellular experiments and transcriptomic analyses revealed the underlying mechanisms by which the material promotes cartilage regeneration. This artificial proteoglycan assembly can effectively recruit bone marrow-derived mesenchymal stem cells and, by downregulating the expression of genes related to calcium signaling pathways and extracellular matrix–receptor interactions, reduce cytoplasmic calcium ion concentrations and decrease the clustering of integrin β1, thereby inhibiting the tendency toward endochondral ossification. This creates a microenvironment more conducive for stem cells to differentiate into cartilage rather than bone, promoting the secretion of glycosaminoglycans and type II collagen while reducing the production of type I and type X collagen, which helps form transparent cartilage-like tissue.
Reference News:
DOI: 10.1038/s41467-025-67035-6






